Research



Biophotonics: Machine learning and nanophotonics for label-free molecular-to-cellular detection
Different cell species have unique Raman “fingerprints” that describe their chemical composition. Raman spectroscopy provides comprehensive information of a whole cell, spanning not only its genes and transcripts but also the proteome and the vast landscape of the metabolome. To provide some quantitative estimates, bacterial cells contain around 4,000 protein-coding genes, eukaryotic cells more than 6,000, and the human genome contains around 20,000 protein-coding genes and 200,000 transcripts; each of these can be embedded in one single-cell Raman spectrum. Raman spectroscopy also
provides further information about the approximately 1 million proteoforms of the human proteome, including their intricate post-translational modifications. Naturally, a Raman spectrum from a single cell will contain a convolution of each of these molecular signatures. Still, this whole-cell fingerprint can complement traditional omics by defining the collective molecular composition instead of solely the genomic/transcriptomic/proteomic activity. Furthermore, compared to conventional culture-based assays, Raman spectroscopy provides benefits of high speed, low limits of detection and reduced sample volume, as culturing can take days to weeks and more than 98% of bacteria and some yeast strains 22 are also non-culturable in labs. Finally, Raman provides a label-free approach, in contrast to immunoassay-based techniques (such as commonly used enzyme-linked immunosorbent assay), nucleic acid detection-based methods (such as PCR); and is also non-destructive, in contrast to mass spectrometry-based protein and metabolite detection. These merits of Raman spectroscopy can enable potential high throughput and cost-effective phenotyping, with promise for point-of-care clinical diagnostics and on-site environmental monitoring.
We are developing surface-enhanced Raman spectroscopy (SERS) methods, assisted with machine learning (ML) models, to enable interpretable insights into the transcriptome, proteome, and metabolome at the single-cell level. We pioneer approaches in nanophotonics – including plasmonics, metamaterials, and metasurfaces – to enhance Raman scattering for rapid, strong label-free spectroscopy. We also develop ML approaches for precise and interpretable spectral analysis, including neural networks, perturbation and gradient algorithms, and transfer learning.
Current translational efforts in our lab including using AI-enabled single-cell Raman for bacterial antibiotic susceptibility predictions, cancer diagnostics, and immunotherapy/cell-therapy efficacy and toxicity predictions. We are also exploring exciting prospects for the future of single-cell Raman spectroscopy, including Raman instrumentation, self-driving labs, Raman data banks, and machine learning for uncovering new biological insights.
Biomolecular analysis methods are foundational to advancing personalized and preventative medicine, accelerating disease diagnostics, and monitoring the health of organisms and ecosystems. Our lab is developing high quality factor (High Q) metasurfaces for rapid, label-free optical-based detection and characterization of critical biorecognition elements in health and in the environment. Our lab developed the first high-Q metasurfaces” – nanostructured silicon films that strongly amplify light while controlling the direction of its transmission. We also showed that these metasurfaces could enable high-Q’s in subwavelength mode volumes. This foundational technology enhances electromagnetic fields in ultra-small, molecular-to-cellular-scale volumes, enabling detection of low-abundance biomarkers without need for lengthy culturing or biomarker replication steps. These metasurfaces also create an easily-imaged pixel array to detect many target molecules at once. Currently, metasurfaces are patterned at sensor densities of 5 million per square centimeter, comparable to over 31,000 96-well plates.
We are now integrating these sensors with microfluidic platforms for DNA and RNA detection and sequencing. One exciting application is enabling quantitative measurements of biodiversity. Environmental DNA – the genetic material released from organisms into the environment – is a potential route to detection of harmful, invasive, or endangered species. Current approaches for measuring eDNA such as polymerase chain reaction (PCR) and next-generation sequencing require large capital investment, complex sample processing, amplification of select genes to target organismal groups, and molecular labeling, which can take weeks to months to gain results. We are developing high-Q nanophotonics to quantitatively measure eDNA in marine environments in collaboration with the Micheli Group (Stanford Center for Ocean Solutions & the Hopkins Marine Station) and the Palau National Marine Sanctuary. In collaboration with the Monterey Bay Aquarium Research Institute and NOAA, we are also designing our setup for in-line installation with the Environmental Sample Processor (ESP), an automatic water sampler that rides onboard autonomous underwater vehicles (AUVs) capable of in situ measurements in the open ocean.
We are also developing these sensors for sensitive metabolomic detection. We are developing wearable platforms that rapidly, quantitatively, sensitively, and continuously detect metabolite biomarkers including adenosine, dopamine, oestradiol, and cortisol, which have been shown to correlate with sleep, mood, cognitive function and chronic stress.By facilitating continuous, non-invasive monitoring of stress biomarkers, we aim to substantially improve individual health management and reduce the incidence and severity of health conditions for pro-active solutions.
The chirality, or ‘handedness’, of biomolecules can strongly dictate a compound’s activity, efficiency, and safety. Enantiomers often exhibit different toxicological, pharmacokinetic, and physiological properties. Chiral molecules now comprise more than a third of agrochemicals and more than half of pharmaceuticals. Yet, the high cost associated with separation and asymmetric synthesis procedures leads commercial markets to predominantly sell racemic mixtures: over 90% of chiral molecules are sold as mixtures, despite adverse side effects, potential toxicity, and delayed onset times in pharmaceuticals and other biomolecules. Our lab is developing more facile methods for stereoselectivity to bring more effective medications and agrochemicals to the public, enable agile response to medical and agrochemical crises, and increase supply chain efficiency for essential biochemical production.
Because enantiomers share nearly all physical scalar properties, separation often demands strong interaction with an additional, compatible chiral agent or substrate. In industry, stereopure products are generally produced through one of two routes: asymmetric synthesis of one enantiomer or resolution of racemic mixtures. While asymmetric synthesis is more reagent and time efficient, the approach remains limited in scope and struggles to produce sufficient purity, in addition to the cost of designing, producing, and then removing precious metal catalysts. Chiral resolution after production through chromatography or crystallography is the more common approach, but the cost and infrastructural difficulties of these routes pose significant barriers to scalable manufacturing. Improvements to stereopure production technology hold the potential to reduce the costs of chemical synthesis, moderate industrial environmental impact, and generate more effective pharmaceuticals and other biotechnological materials.
Our lab is developing a new, optics-based method for enantioselective synthesis of chiral molecules – from small molecules to larger oligo strands. By developing optical nanostructures that enhance chiral light-molecular interactions, we are using light as a versatile, low-cost reagent in enantiopure chemical synthesis. Through a combination of state-of-the-art computation, AI, CMOS-compatible fabrication, and molecular synthesis, we aim to address key conceptual gaps regarding chiral light-matter interactions, chiral-induced spin-selectivity, and advance enantiopure synthesis techniques applicable to a broad range of pharmaceutical and agrochemical compounds.
We are using photonic tools to understand how coral reef ecosystems function, and also why they are so threatened by global warming. Coral reefs are solar-powered: photosynthetic creatures harness sunlight to provide energy to the biodiverse reef ecosystem. These creatures have evolved intricate biophotonic adaptations to harness solar power. But heat and light stress can actually kill corals, a problem which may be exacerbated by the highly efficient light-focusing biophotonic adaptations. We are applying tomography and electron microscopy to image coral reef creatures, fluorimetry and reflectance spectroscopy to characterize their optical properties, FTIR and Raman to probe their material compositions, and finite-difference time-domain optical models to simulate the propagation of light through tissue. Taken together, this work aims to characterize new natural biophotonic technologies and explain why heat and light stress are so damaging to corals– with the goal of charting a course to a more resilient, sustainable future.



Active and electro-optically-tunable nanophotonics
Using photons in conjunction with electrons for information processing can open up new possibilities for computing devices operating at the speed limit of the universe. However, optical devices have traditionally been subject to diffraction limits and have remained bulky, limiting their opportunity for dense integration in networks. Metasurfaces are a nanophotonic approach to scaling down the size of optical components by using arrays of subwavelength nanoantennas to control the phase, polarization, and amplitude of light. Our lab is developing highly resonant metasurfaces by making subtle perturbations to periodic structures, breaking translational symmetry and giving rise to high quality factor (high-Q) resonances. We use the resulting enhanced light-matter interactions to realize nanophotonic devices for optical computing, communication, and quantum information applications.
See related research on high-Q metasurfaces with applications in Health here!
Traditional passive metasurfaces are limited to perform a single function by their architecture. However, active control over the spatial propagation of light is needed for applications in spatial computing technologies, such as LiDAR, AR/VR, LiFi, etc. Reconfigurable metasurfaces tackle this by integrating an optically active medium to dynamically change their nanoantenna after fabrication to perform different functions such as tunable beamsteering, beamsplitting, and lensing, among others. By further integrating high-Q resonances into these device designs, we can take advantage of the highly enhanced near-fields to improve the strength of otherwise weak light-matter interactions and the highly sensitive resonant features in the far field to control device performance with high efficiency.
Integrating high-Q resonances into silicon devices, such as a metasurface lens, allows us to efficiently utilize the weak nonlinear Kerr effect inherent to the silicon. Changing the input laser power or temperature the device operates at demonstrates significant shifts of the high-Q resonance, corresponding to modulation of the focal length and intensity and achieving a power-limiting metasurface.
We further design and fabricate electro-optically reconfigurable metasurfaces to completely control the outgoing optical wavefront by applying individual electric biases to constituent nanoantennas. Our highly resonant silicon-on-lithium niobate metasurface design allows us to do this at low voltages and high efficiency, compared to other reconfigurable metasurfaces. Using reconfigurable metasurfaces, we hope to replace large, bulky optical components in spatial light modulation devices with a compact, lightweight nanophotonic platform.
Single photons are promising sources for quantum computation, but the creation and manipulation of single photons is difficult to reliably control. High-Q metasurfaces allow us to control the electric field near the photon source, giving us the ability to enhance emission, decrease decoherence, and control the emitted polarization and phase. Unique to dielectric metasurfaces, we can design electric and magnetic resonances that are tuned to couple strongly with the spin of a particle, in addition to generating the strong light confinement that enhances this interaction. This work enables us to better understand emerging quantum emitters and design new platforms for quantum computation.
Semiconductor excitations – termed excitons – are the primary quasi-particles used in optoelectronic applications such as light-emitting diodes, solar cells, or single-photon sources. Tailoring excitons has so far been limited to the native length scales of excitons of a few nanometers by molecular and atomic engineering of the semiconductor. However, by hybridizing optically-active semiconductor with cavity-confined photons, we can tailor the absorptive and emission properties by accessing a new primary excitation, the exciton-polariton. Our group works towards engineering exciton polaritons via the photonic control inherent to nanophotonic elements such as dielectric metasurfaces and plasmonic gap cavities. Ultimately, our efforts will provide new ways to engineer the optical properties of semiconductors via long-range tuning of photonic effects.



Photocatalysis: Electrifying the Chemical Manufacturing Industry
Chemical manufacturing is critical for industries spanning construction, clothing, plastics, pharmaceuticals, food, and fertilizers. These processes rely on catalysts, typically metal nanoparticles, to accelerate reaction rates, yet they remain among the most polluting and energy- demanding industrial practices. More effectively controlling these chemical transformations requires bridging the length -scale between a catalyst’s atomic-scale structural features that influence dynamics and the macroscale extrinsic parameters that can be controlled (e.g., illumination, temperature, pressure).
Our research focuses on enabling sustainable chemical production with atomically architected nanoparticle heterogeneous photocatalysts that precisely control optical, electronic, and molecular interactions for high-efficiency and product-selective photochemistry. Plasmons, or collective oscillations of conduction electrons within a metal, offer a solution for overcoming this size mismatch. Optical excitations of plasmon resonances in a metal nanoparticle can create nanoscopic regions of high electromagnetic field intensity that can modify electronic and molecular energy levels, enabling access to excited-state dynamics, and open new reaction pathways that are impossible to achieve under typical conditions.
The challenge our group seeks to address is understanding how these ultrafast and nanoscopic optical interactions correspond to macroscopic changes in chemical activity using a multiscale approach. By revealing the multiscale photochemical processes that span Angstroms to centimeters and picoseconds to minutes, our catalyst design and characterization could overturn the century-old empirical approach of catalyst development, and inform a new generation of sustainable, light-driven catalysis.
Metals that are traditionally considered plasmonic, i.e. have strong plasmon resonances in the visible wavelength, such as Ag and Au, are excellent at concentrating and absorbing incoming illumination but are not very catalytically active for many reactions. On the other hand, metals commonly used for their catalytic properties, e.g. Pd and Pt for hydrogenation chemistries, have optimal electronic properties for activating certain reactant molecules at their surface but do not support plasmons in the visible and thus cannot be efficiently optically excited by solar energy. However, combining both types of metals into the same bimetallic catalyst system allows for both efficient light absorption and chemical bond activation.
Our work explores the bimetallic catalysts that incorporate both a plasmonic and catalytic metal to synergistically capture light and use it to drive chemistry with high activity, selectivity, and stability. We synthesize a variety of bimetallic nanoparticles consisting of a plasmonic and catalytic metal in well-controlled morphologies, compositions, and configurations, then characterize their catalytic and material properties on both the ensemble and single-particle level. Our catalysts have demonstrated the ability for photoexcitation to overcome the common trade-off between activity and selectivity in heterogeneous catalysis in a range of hydrogenation reactions – including ammonia synthesis, ethylene synthesis, and even steel manufacturing.
A major challenge to industrial decarbonization is replacing fossil fuels with renewable energy sources. Electrochemistry offers a pathway to industrial electrification. Unlike electrical heating, this approach uses electrons to break and make chemical bonds – directing electrons right where they’re needed, allowing increased efficiency and selectivity and avoiding challenges associated with heat transfer. However, key electrochemical reactions such as CO2 reduction,, water splitting, and ammonia synthesis, require the transfer of multiple electrons, resulting in high overpotentials and significant kinetic losses. As a result, electrosynthesis often requires high energy input.
Photo-electrocatalysis offers a sustainable pathway to drive such multi-electron chemical reactions with renewable electricity. Light, supplied by sunlight or efficient light-emitting diodes (LEDs) can, provides an opportunity to manipulate reaction pathways to overcome reaction barriers and lower the onset potentials of electrochemical reactions. Additionally, plasmonic electrocatalysts have been shown to lead to different reaction products, allowing electrocatalysis to be more selective toward certain reactions.
Our lab is developing plasmon photoelectrochemical methods for CO2 utilization, removing hard-to-abate emissions. CO2 capture is looked at as a solution to remove CO2 emissions from sectors that cannot be easily electrified, such as the petrochemical industry, and sectors that emit CO2 process emissions, such as iron and cement production. However, carbon capture is expensive and provides no financial benefit. We are developing ways to capture CO2 and convert it into hydrocarbons to produce chemical feedstocks and fuels. Currently, our work is focused on photoelectrochemical conversion of CO2 in flue gas into ethylene, one of the most widely used and valuable petrochemicals. Plasmon excitation has been shown to produce high-value multicarbon (C2+) products under illumination, even on catalysts that normally cannot perform electrochemical C-C coupling. We are synthesizing plasmonic Cu nanoparticles and incorporating them into our home-built gas diffusion electrode. We investigate how the interplay between plasmon excitation, applied potential, and temperature can be used to increase conversion and selectivity. Using a combination of gas chromatography, surface-enhanced Raman spectroscopy, and DFT simulations, we gain the mechanistic understanding necessary for efficient and selective photo-electrochemical CO2 conversion of flue gas streams.
Transmission electron microscopy (TEM) techniques enable imaging and chemical and optical characterization of nanomaterials with atomic-scale resolution. Our facilities are equipped with an environmental optically-coupled transmission electron microscope (E-OTEM), wherein both light and reactive gases can be introduced into the microscope, which allows observation of gas-phase, light-driven chemical transformations in real-time.
We use E-OTEM to monitor hydrogenation reactions catalyzed by our bimetallic plasmonic and single-atom catalysts, tracking how reaction outcomes are correlated to changes in nano-to-atomic-scale material properties such as size, shape, composition, and crystallinity. Our findings reveal that illumination of plasmonic catalysts can drive site-selective chemistry that is distinct from what is favored in the dark.

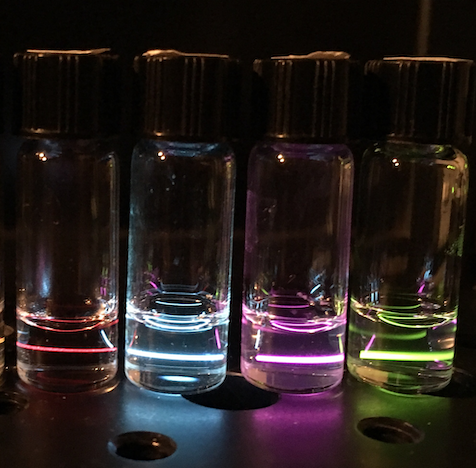

Extreme energy-shifting nanoparticles: Upconversion and Scintillation
Lanthanides and rare earth metals have the ability to convert light between different spectral regions with sharp absorption and emission peaks. By doping them into ceramic hosts to make lanthanide-doped nanoparticles (LNPs), we can achieve optical nanoparticles that don’t photobleach or photoblink and can achieve both downconversion and upconversion. By customizing the specific dopants, host lattice compositions, and nanoparticle size, we can make LNPs useful for a wide variety of imaging applications, including bioimaging and high energy radiation scintillators.
Upconverting lanthanide-doped nanoparticles (UCNPs) can emit at shorter wavelengths than they absorb, due to the ladder-like energy structure and long-lived energy states of certain rare earth elements. With a variety of dopants, visible light is emitted across a rainbow of colors when irradiating with near-infrared (NIR) light. NIR excitation penetrates biological tissue more easily and deeply than ultraviolet or visible light, and the UCNP’s size and biocompatibility make them excellent candidates for non-perturbative bioimaging. UCNPs have also been shown to respond colorimetrically to externally applied force or pressure. They fill an important gap in the landscape of tools for measuring biological forces. Combined with their stable optical properties and functionalizable surface, UCNPs can become a powerful tool for rapid and minimally invasive bioimaging methods for multiple biological systems and various length scales.
In our lab, we are developing UCNPs as biomechanical force sensors. We tune their sensitivity with different host lattice, dopant compositions and nanoparticle sizes. We are also developing polymeric delivery formats for systems from the millimeter scale, in the mouse gastrointestinal tract, to the microscopic scale, with pharyngeal pumping in C. elegans worms, to the single particle level, measuring forces between individual immune cells.
To quantify and calibrate UCNPs’ force-spectral response, we have two primary measurement methods. We use a Diamond Anvil Cell to demonstrate the sensitivity and cyclability of force-spectral response in ensembles of nanoparticles, and a tandem Atomic Force and Confocal Microscope to simultaneously exert force on single particles or particle-embedded polymer units while collecting optical emission signal.
Radiation imaging and therapy is a cornerstone of cancer treatment alongside surgery, chemotherapy, and immunotherapy. Photodynamic therapy (PDT) offers a minimally invasive approach to precision radiation therapeutics with fewer side effects. However, shallow tissue penetration depth and high radiation dose limit PDT efficacy to superficial tumors. X-ray induced photodynamic therapy (X-PDT) combines PDT principles with deep tissue penetration of X-rays, offering a promising approach with minimal radiation toxicity. In X-PDT, radioluminescent nanoparticles known as nanoscintillators convert X-ray energy to activate photosensitizer molecules (PS) to generate reactive oxygen species. These reactive oxygen species are crucial for cancer cell death, enabling targeted treatment of deep-seated or hypoxic tumors.
Our lab is developing stable, biocompatible “nanoscintillators” with bright and persistent luminescence. Our programmable “super scintillators” are multicomponent nanocarriers for X-PDT and imaging probes that integrate advances in design, synthesis, and optimization. We are pursuing three main objectives: (1) engineering nanoscintillators with modular emission wavelengths and long decay lifetimes; (2) enhancing ROS generation and therapeutic efficacy by conjugating nanoscintillators with PSs and active targeting ligands; and (3) assessing in vitro and in-vivo efficacy using tissue and animal models to advance combined imaging and therapy for hard-to-treat cancers.

